µGCSE:Density
10 quick questions - for GCSE and iGCSE
|
10 minutes maximum! Can you do it in 5? |
||||||||||||||||||
1. Which of these is the correct formula for density?
| ||||||||||||||||||
Q2+3: This metal cube of side 2cm has a mass of 32g. |
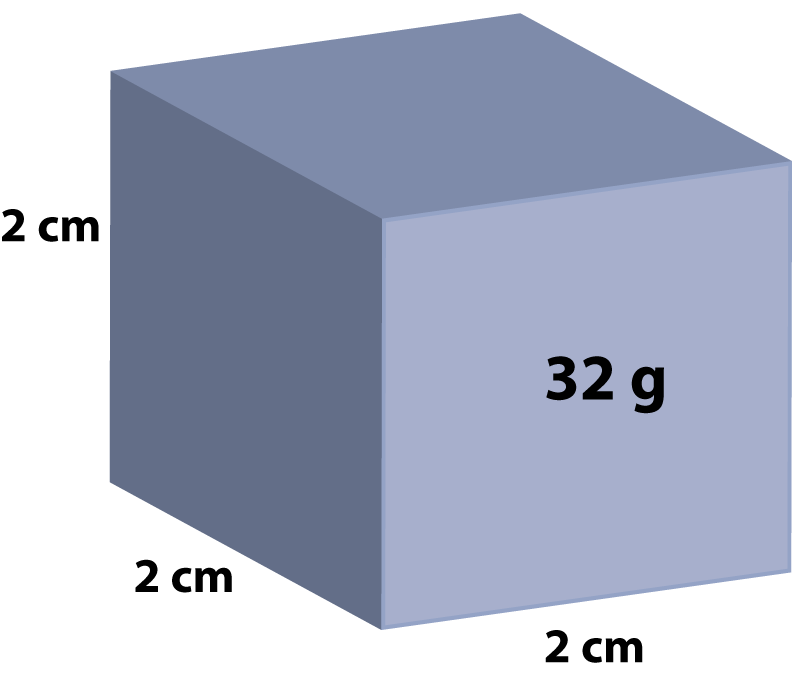 |
|||||||||||||||||
2. The density is ...
| ||||||||||||||||||
3. If you heated the cube it would expand a little. This would make ....
| ||||||||||||||||||
Q 4-6. These questions are about the densities of different substances. What are the missing values? |
||||||||||||||||||
|
||||||||||||||||||
7. Floating or sinking depends on density. The density of water is 1 g/cm3. The density of this block is is 1.1 g/cm3.
|
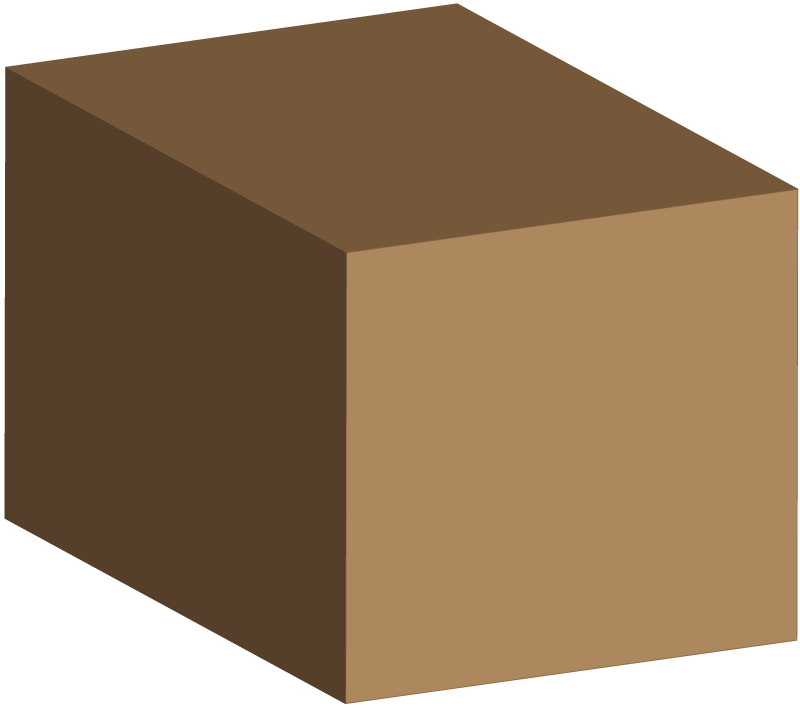 |
|||||||||||||||||
This means that if you put this block in water it will...
| ||||||||||||||||||
Q8-10. A green ball has been put into the measuring cylinder shown, and the water level has risen. |
||||||||||||||||||
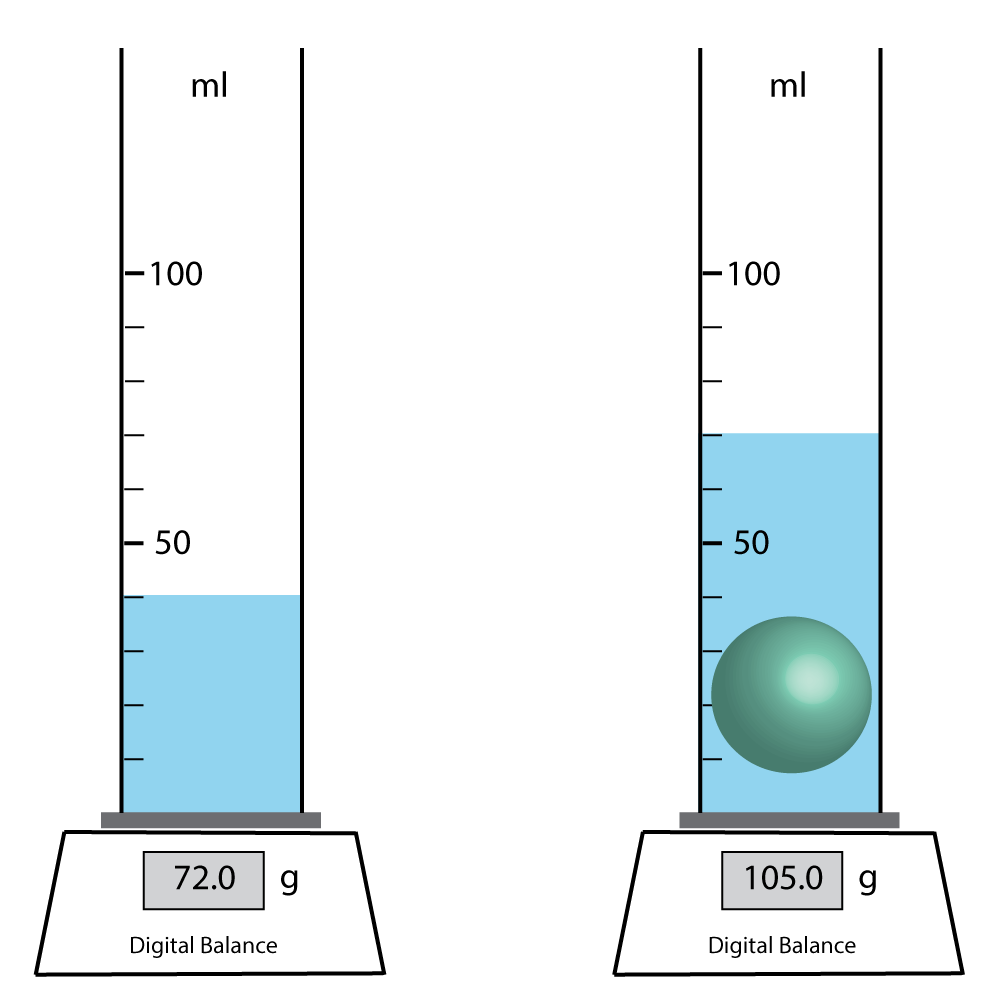 |
||||||||||||||||||
8. The water has been...
| ||||||||||||||||||
9. Using measurements from the diagram above, what is the density of the ball?
| 10. If the ball is cut in half and the experiment repeated, the density will . | |||||||||||||||||