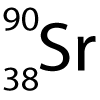|
10 minutes maximum! Can you do it in 5?
|
1. Which particles are in the centre of an atom?
- A. protons and electrons.
- B. neutrons and electrons.
- C. neutrons and protons.
- D. protons, neutrons and electrons.
| |
2. What is the name of the centre part of an atom, and what orbits around this?
|
Centre part |
In orbit |
A |
nucleus |
protons |
B |
nucleole |
electrons |
C |
nucleus |
electrons |
D |
nucleole |
protons |
| |
Q3-6. Which of these particles.... |
|
3. ..has a negative charge. |
|
4. ..has a mass of 1 atomic unit and a positive charge. |
|
5. ..has a neutral charge. |
|
Here is a diagram of an atom of Beryllium.
It has the symbol:
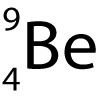
|
 |
6. What does the top number represent?
- A. The number of protons.
- B. The number of protons plus electrons.
- C. The number of neutrons.
- D. The total mass of the nucleus.
| |
7. What does the bottom number represent?
- A. The number of protons.
- B. The number of protons plus electrons.
- C. The number of neutrons.
- D. The total mass of the nucleus.
| |
8-10: Strontium isotopes:
The symbol for the radioactive isotope strontium-90 is shown here. |
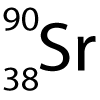 |
8. How many protons (p) and neutrons (n) does it have?
- A. 90 p, 90 n
- B. 38 p, 90 n
- C. 52 p, 38 n
- D. 38 p, 52 n
| |
9. Two different isotopes will have...
- A. the same number of protons but a different number of neutrons.
- B. the same number of neutrons but a different number of protons.
- C. the same number of protons but a different number of electrons.
- D. the same number of electrons but a different number of protons.
| |
10. Question 8 descibes an isotope of strontium. Which of these describes the nucleus of a different isotope of strontium?
- A. 90 p, 91 n
- B. 39 p, 52 n
- C. 38 p, 53 n
- D. 53 p, 38 n
| |
|