Chemistry µGCSE:Properties
10 quick questions - for GCSE and iGCSE
10 minutes maximum! Can you do it in 5? |
||||||||||||||||||||||||
| 1. Sodium chloride has a high melting point. | 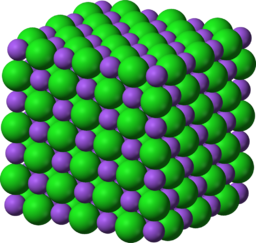 |
|||||||||||||||||||||||
This is because it requires a lot of energy to break the ...
| ||||||||||||||||||||||||
2. Which of the following substances could represent the giant covalent substance silicon dioxide?
| ||||||||||||||||||||||||
3. A covalent bond is the ...
| ||||||||||||||||||||||||
Q4-6: Properties of substances P to S are given in the table below:
|
||||||||||||||||||||||||
4. Which substance has metallic bonding? |
||||||||||||||||||||||||
5. Which substance has ionic bonding? |
||||||||||||||||||||||||
6. The substance(s) with a giant structure are ...
| ||||||||||||||||||||||||
| 7. Carbon dioxide has a low melting point. | 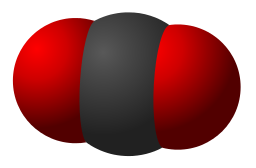 Jacek FH | CC-BY-SA 3.0 |
|||||||||||||||||||||||
The reason for this is because ...
| ||||||||||||||||||||||||
8. Graphite is a good electrical conductor because it has ...
| ||||||||||||||||||||||||
9. Diamond is a very hard substance which is used in cutting tools because it has ...
| ||||||||||||||||||||||||
10. Aqueous or molten ionic substances conduct electricity because they have ...
| ||||||||||||||||||||||||
Salt Flats, Bolivia | otrebskamarta | Pixabay