10 minutes maximum! Can you do it in 5? |
1. Ionic bonding involves the ...
- A. sharing of electrons between metal atoms
- B. sharing of electrons between non-metal atoms
- C. transfer of electrons from metal to non-metal atoms
- D. transfer of electrons from non-metal to metal atoms
| |
2. Covalent bonding involves ...
- A. sharing of electrons between metal atoms
- B. sharing of electrons between non-metal atoms
- C. transfer of electrons from metal to non-metal atoms
- D. transfer of electrons from non-metal to metal atoms
| |
Q3+4: Sodium burns in oxygen to form sodium oxide:

|
|
3. In this reaction the sodium atom ...
- A. loses one electron and forms a positive ion with a 1+ charge
- B. loses one electron and forms a negative ion with a 1- charge
- C. gains one electron and forms a positive ion with a 1+ charge
- D. gains one electron and forms a negative ion with a 1- charge
| |
4. In this reaction the oxygen atom ...
- A. gains one electron
- B. loses one electron
- C. gains two electrons
- D. loses two electrons
| |
5. Hydrogen and chlorine atoms combine to form hydrogen chloride gas. In this reaction the ...
- A. hydrogen atom loses electrons and chlorine atom gains electrons
- B. chlorine atom loses electrons and hydrogen atom gains electrons
- C. chlorine atom shares one pair of electrons with the hydrogen atom
- D. chlorine atom shares two pairs of electrons with the hydrogen atom
| |
6. Diagrams of magnesium and fluorine atoms are shown below:
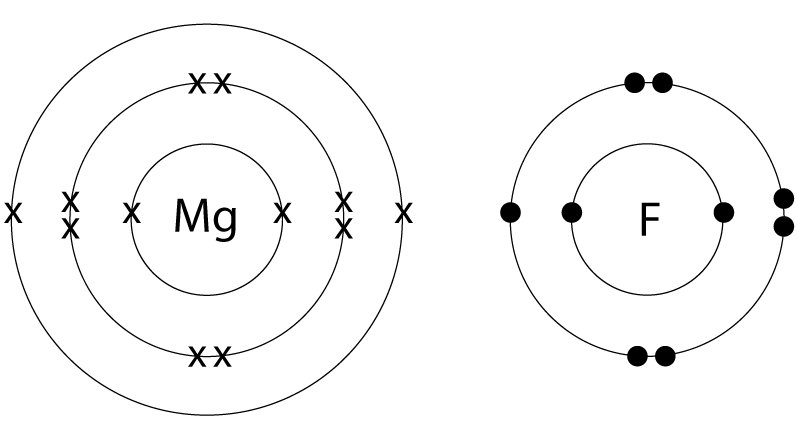
Select the dot and cross diagram which correctly represents the bond formed between magnesium and fluorine (outer shells only are shown). |
|
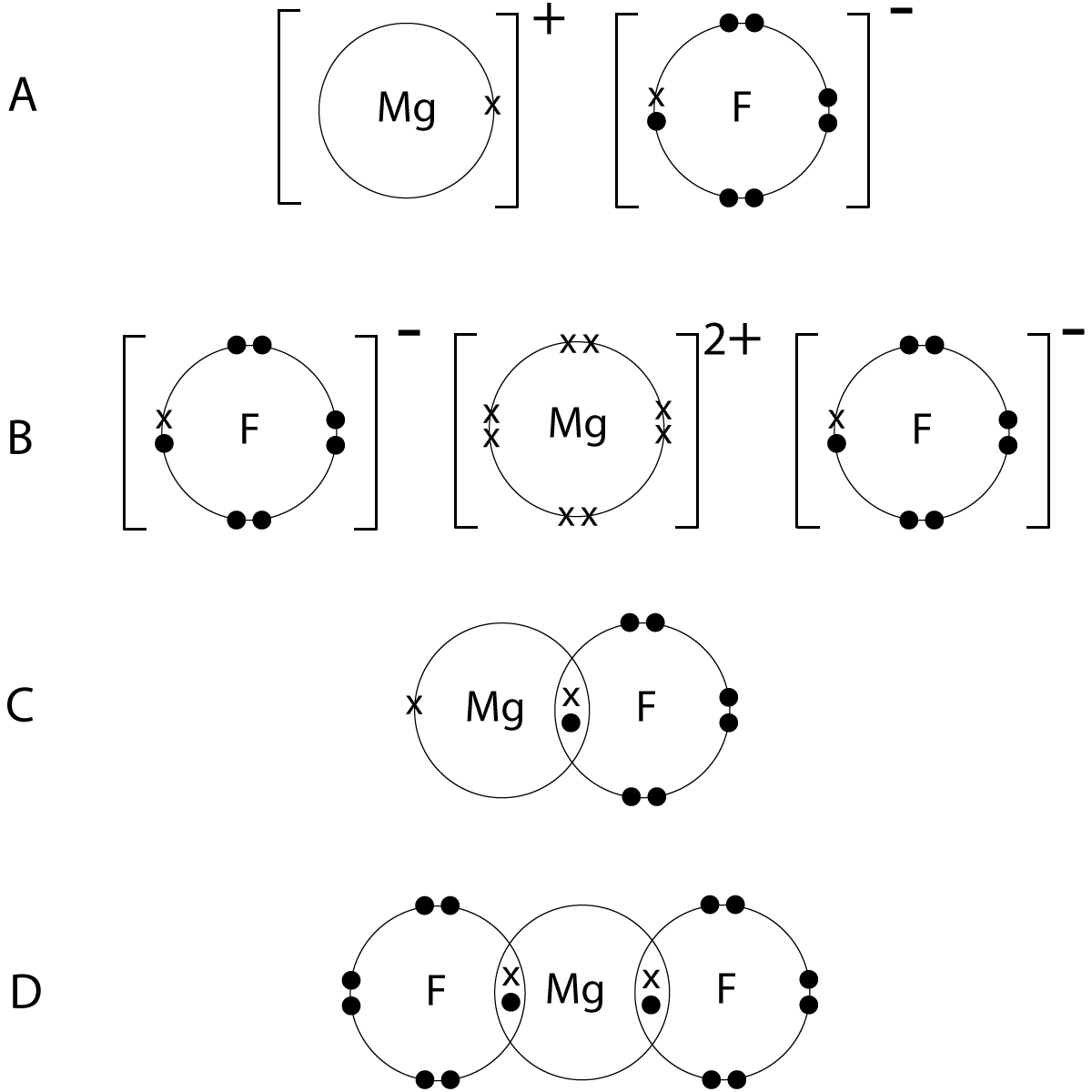 | |
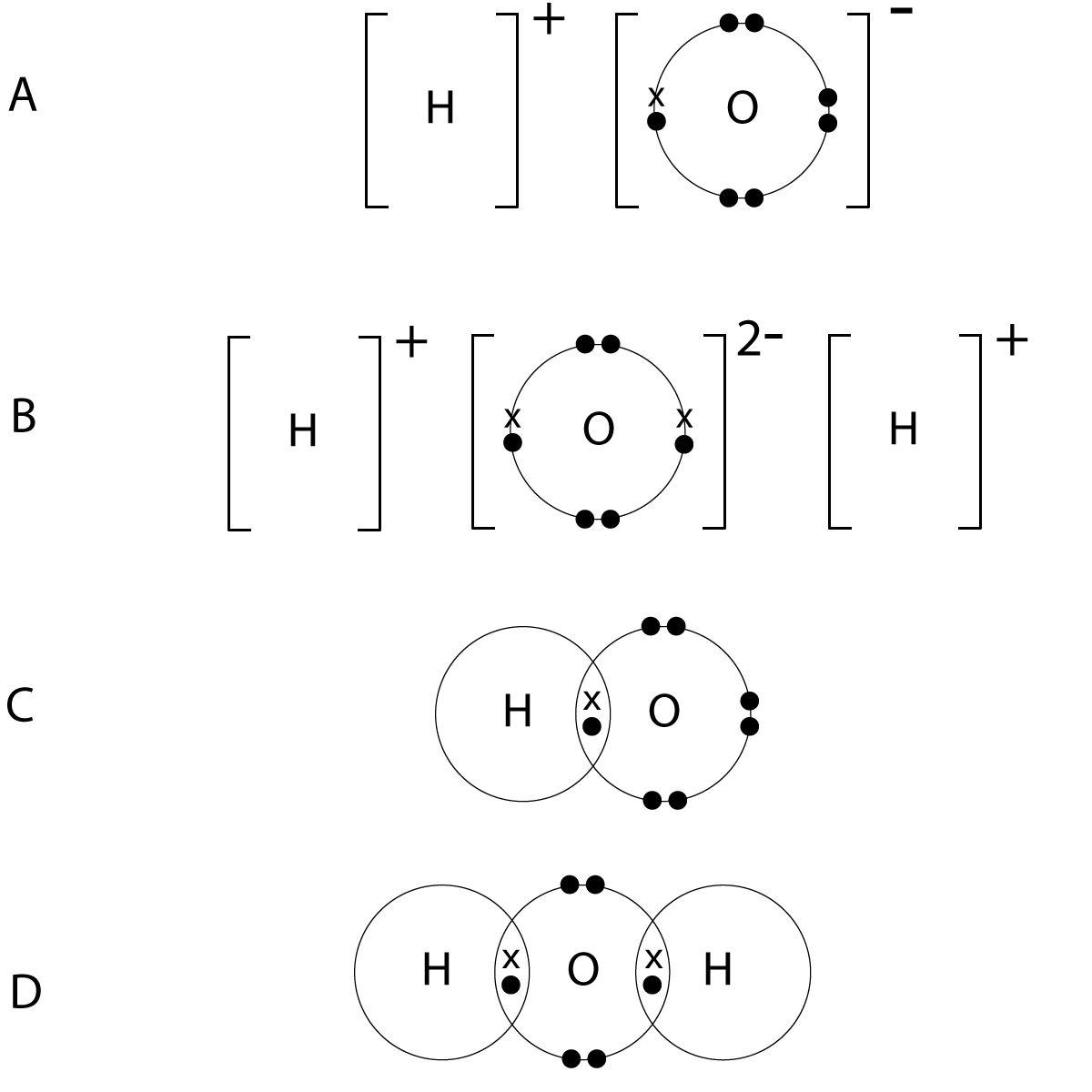
7. Hydrogen reacts with oxygen forming water. Select the dot and cross diagram which correctly represents the bonding in water (outer shells only):
| |
8. When magnesium reacts with sulfur to form magnesium sulfide the electronic configuration of ...
- A. magnesium changes from 2,8,2 to 2,8 and an Mg+ ion is formed
- B. magnesium changes from 2,8,2 to 2,8,1 and an Mg2+ ion is formed
- C. sulfur changes from 2,8,6 to 2,8,7 and an S- ion is
- D. sulfur changes from 2,8,6 to 2,8,8 and an S2- ion forms
| |
9. The two atoms in a molecule of nitrogen gas, N2, bond together by ...
- A. transferring three electrons from one nitrogen atom to the other
- B. sharing three pairs of electrons
- C. sharing two pairs of electrons
- D. sharing one pair of electrons
| |
| 10. The dot and cross diagram which represents the bonding in a molecules of carbon dioxide is: 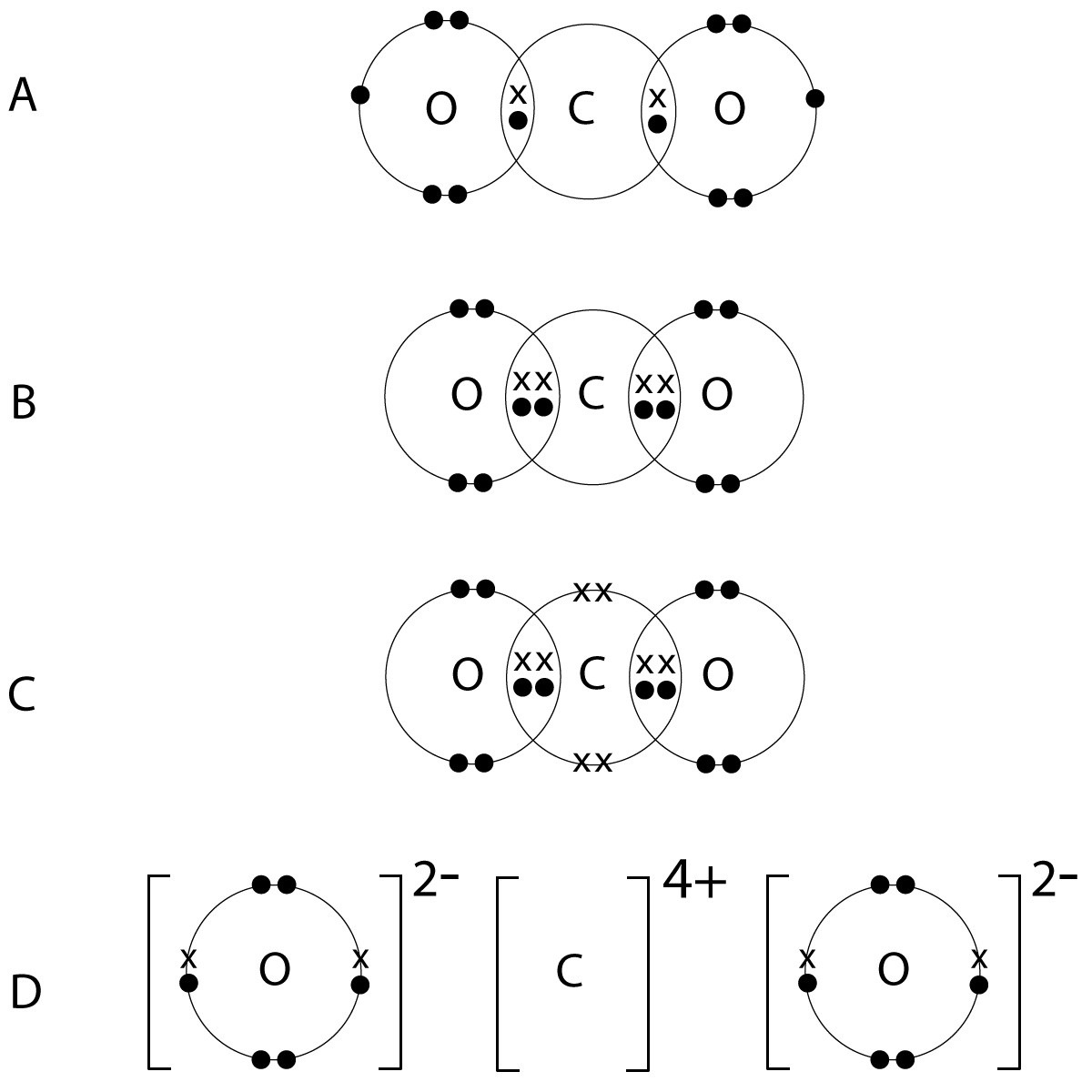
| |
|
