Chemistry µGCSE:Atomic Structure
10 quick questions - for GCSE and iGCSE
10 minutes maximum! Can you do it in 5? |
|||||||||||||||||||||||||
|
1. A diagram of an atom of lithium is shown. |
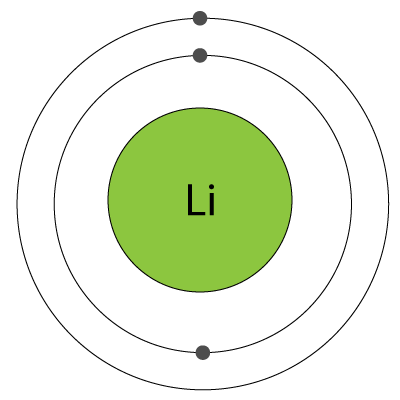 |
||||||||||||||||||||||||
What is the name of the central part of the atom and which sub-atomic particles does it contain?
| |||||||||||||||||||||||||
Q2-4 Which sub-atomic particle has ... |
|||||||||||||||||||||||||
2. ...a relative mass of one atomic mass unit and no charge? |
|||||||||||||||||||||||||
3. ...a negative charge? |
|||||||||||||||||||||||||
4. ...a relative mass of almost zero (1/1836 atomic mass units)? |
|||||||||||||||||||||||||
5. Atoms have no overall charge because the number of ...
| |||||||||||||||||||||||||
6. The electronic configuration of an atom with 16 electrons is ...
|
|||||||||||||||||||||||||
Q7-9 Use the information given in the table below to fill in the missing information for each atom:
|
|||||||||||||||||||||||||
10. Information on four particles, W, X, Y and Z is given below. Which are isotopes?
|
|||||||||||||||||||||||||
| |||||||||||||||||||||||||