10 minutes maximum! Can you do it in 5? |
1. Properties of four substances are given in the table below. Which substance is a metallic element?
| |
Melting point /°C |
Electrical Conductivity.. |
| ..when solid |
..when liquid |
| A |
-26 |
low |
low |
| B |
801 |
low |
high |
| C |
3246 |
high |
low |
| D |
1436 |
high |
high |
| |
2. A metallic bond is the ...
- A. mutual attraction of two nuclei for a shared pair of electrons
- B. transfer of electrons from one atom to another
- C. attraction of positive ions for a ‘sea’ of delocalized electrons
- D. attraction of positive ions for negative ions
| |
3. Metals conduct electricity because they contain ...
- A. delocalized electrons which are free to move
- B. delocalized electrons which are unable to move
- C. ions which are free to move
- D. ions which are in fixed positions
| |
4. Metals are malleable because ...
- A. positive and negative ions are in regular positions and can slide over each other
- B. positive and negative ions are in regular positions and cannot move
- C. atoms are arranged in layers so can slide over each other
- D. atoms are arranged in layers and cannot move
| |
| 5. The position of metal Q in the reactivity series is shown below:
K Na Mg Fe (H) Q Cu
Which statements about Q and its oxide are correct? |
|
| |
Reaction of oxide of Q with carbon |
Reaction of metal Q with dilute acid |
| A |
no reaction |
no reaction |
| B |
no reaction |
reacts, hydrogen formed |
| C |
oxide reduced |
no reaction |
| D |
oxide reduced |
reacts, hydrogen formed |
| |
6. Three metals are extracted as shown in the table below:
| Metal |
Method of Extraction |
| R |
Occurs naturally as the metal |
| S |
Electrolysis of molten compound |
| T |
Heat metal oxide with carbon |
|
|
The order of reactivity of these metals, most reactive first is ...
- A. R, S, T
- B. R, T, S
- C. S, T, R
- D. T, S, R
| |
7. Iron is extracted from iron(III)oxide in the Blast Furnace by heating with carbon monoxide. The equation for this reaction is:
Fe2O3 + 3CO → 2Fe + 3CO2
The substance reduced in this reaction is ... |
|
- A. iron(III)oxide
- B. carbon monoxide
- C. iron
- D. carbon dioxide
| |
8. Aluminium cannot be extracted from its ore by heating with carbon because aluminium is ...
- A. less reactive than carbon
- B. more reactive than carbon
- C. extracted by electrolysis
- D. corrosion resistant
| |
9. Aluminium is extracted by the electrolysis of molten aluminium oxide (Al2O3) using the apparatus shown.
Which row below correctly shows the half equations for the reactions occurring at the electrodes? |
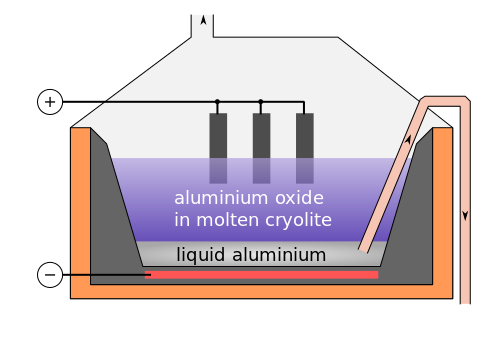
Cepheiden | CC 3.0 |
| |
Equation at cathode |
Equation at anode |
| A |
2O2- → O2 + 4e- |
Al3+ + 3e- → Al |
| B |
O2- → O + 2e- |
Al3+ + 3e- → Al |
| C |
Al3+ + 3e- → Al |
O2- → O + 2e- |
| D |
Al3+ + 3e- → Al |
2O2- → O2 + 4e- |
| |
10. Which of the oxides MgO, CuO and K2O can be reduced by heating with carbon?
- A. K2O only
- B. CuO only
- C. MgO and K2O only
- D. MgO and CuO only
| |
|
