Chemistry Edexcel iGCSE:States+Separations
Page 2
| 12. A few drops of liquid bromine were placed in the bottom of a gas jar as shown and left for several hours. | 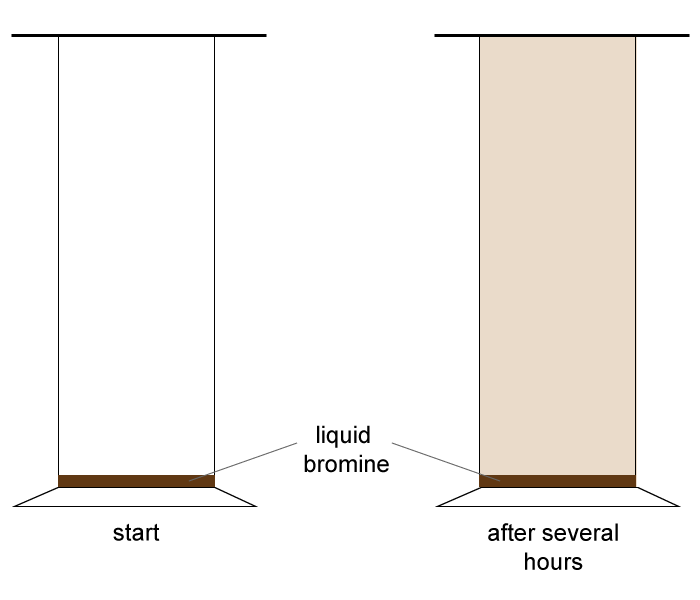 |
|
What two processes occurred in the gas jar during this time?
|
||
13. Which of the following statements is true?
|
||
14. Which method could be used to separate a mixture of salt and water to obtain BOTH parts of the mixture?
|
||
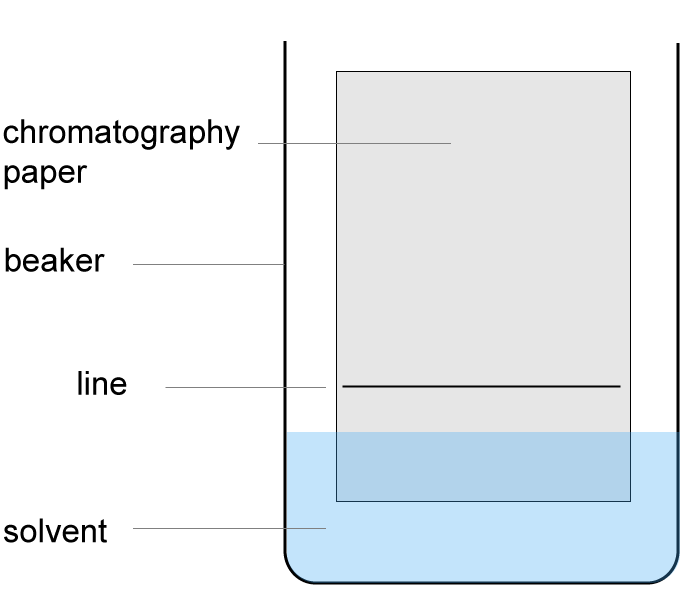
Q15-16:
|
 |
|
15. Where should the student place the food colouring?
|
||
16. Which statement about this experiment is correct?
|
||
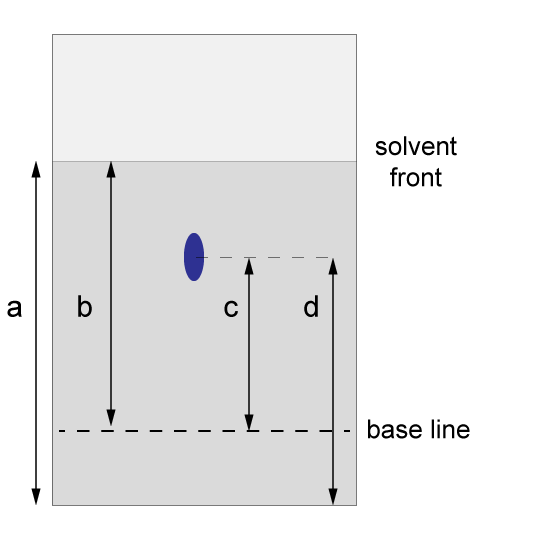
17. A student obtained the following result from an unknown substance. He calculated the Rf value for the spot in order to identify it.
|
 |
|
Which is correct for the calculation of the Rf value?
|
||
Q18-20: |
||
18. ethanol from an aqueous ethanol solution.
|
||
19. excess solid from the mixture formed by reacting excess solid copper(II)carbonate and dilute hydrochloric acid.
|
||
20. solid copper(II)chloride from a copper(II)chloride solution.
|
||