Chemistry Edexcel iGCSE:Gases+Pollutants
Page 1
|
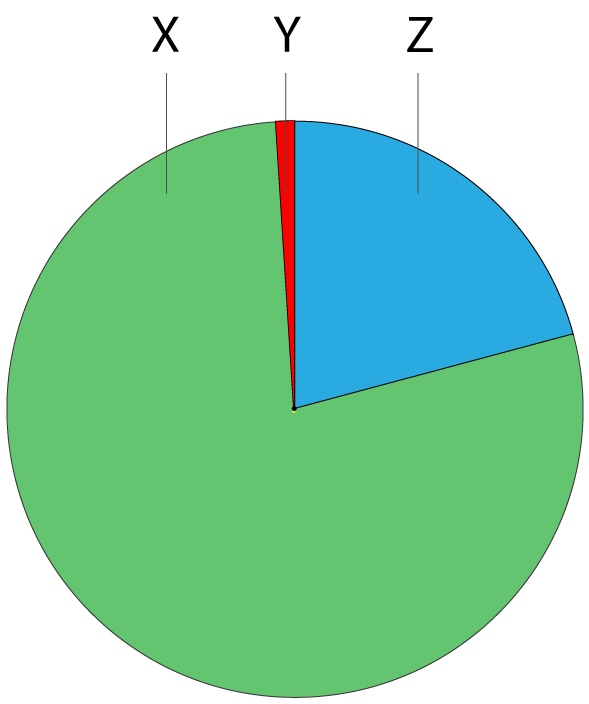
1. The pie chart shows the approximate percentage of the three most abundant gases in dry air.
The gases represented by X, Y and Z are: |
 |
|||||||||||||||||||||
|
||||||||||||||||||||||
2. The test for oxygen gas is …
|
||||||||||||||||||||||
Q3-5: He obtained the following results:
|
|
|||||||||||||||||||||
3. The percentage of oxygen, to the nearest whole number, in the polluted air is..
|
||||||||||||||||||||||
| 4. What would be the effect, if any, on the calculated percentage of oxygen in the air if the student left the experiment set up for longer? | ||||||||||||||||||||||
| 5. What would be the effect, if any, on the calculated percentage of oxygen in the air if the student did not use excess iron wool? | ||||||||||||||||||||||
6. Sulfur was burned in oxygen and the gas produced was added to water and tested with universal indicator (UI). The correct balanced equation for this reaction and colour of universal indicator are: |
||||||||||||||||||||||
|
||||||||||||||||||||||
7. Observations made when magnesium burns in air include..
|
||||||||||||||||||||||
| Q8-9: A sample of clean, dry air is passed over hot copper until all the oxygen in the air has reacted with the copper forming copper(II)oxide. |
||||||||||||||||||||||
|
||||||||||||||||||||||
8. What is the correct equation for the reaction occurring?
|
||||||||||||||||||||||
9. If the volume of air decreased by 20 cm3 what was the starting volume of the air?
|
||||||||||||||||||||||