Chemistry µIB:Resonance (AHL)
Structure 2.2: Resonance and Delocalization
|
Target: 10 Questions in 10 minutes
An IB Chemistry data booklet is helpful |
|||||
1. Which of the following species has a resonance structure?
|
|||||
2. Which species have delocalized electrons in their structure?
| |||||
|
|||||
| 3. The Lewis structure for ozone, O3, is shown here: | 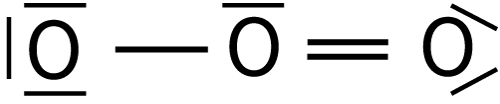 |
||||
Which statement is NOT true for the ozone molecule?
|
|||||
4. What is the correct order of the carbon-oxygen bond length in methanol (CH3OH), methanoate ion (HCOO-) and carbonate ion (CO32-)?
| |||||
5. Which of the following species does NOT show resonance?
| |||||
6. In which of the following species do the S-O bonds have different lengths?
| |||||
7. What is the correct order of the nitrogen-oxygen bond length in NO3- , NO2+ and NO2- ?
| |||||
8. Which of the following statements is NOT true for benzene?
| |||||
9 What is the best description of the bond lengths of the chlorine-oxygen bonds in the chlorate(VII) ion, ClO4- ?
| |||||
10. Which of the following statement are correct for the delocalized electrons in benzene?
| |||||
|
|||||