|
Target: 10 Questions in 10 minutes
An IB Chemistry data booklet is helpful |
|||||||||||||
1. Which of the following can be a unit for the rate constant of a first order reaction?
| |||||||||||||
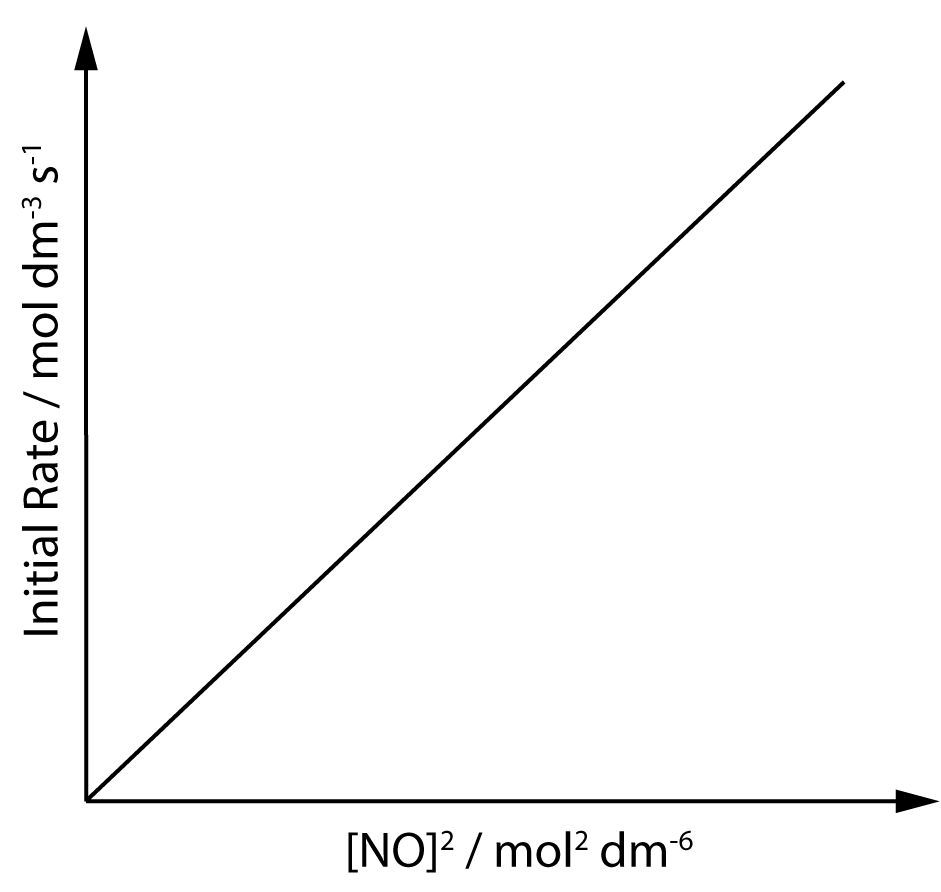
2. For the reaction... 2NO(g) + 2H2(g) → 2N2(g) + 2H2O(g) ..the graph shows how the initial rate of the reaction varies at different concentrations of NO, whilst the concentration of H2 remains constant. What is the order of the reaction with respect to NO? |
 |
||||||||||||
|
|||||||||||||
3. The reaction between sodium thiosulphate and hydrochloric acid is: Na2S2O3(aq) + 2HCl(aq) → S(s) + SO2(aq) + 2NaCl(aq) + H2O(l) Using the following experimental data, what is the rate equation for this reaction?
|
|||||||||||||
| |||||||||||||
4. Two colourless substances, A and B, react to form a coloured substance C according to the equation 2A + B → C. In a series of experiments with different concentrations of A and B, the initial time to form a small about of C is measured as follows:
What is the rate equation for this reaction? |
|||||||||||||
| |||||||||||||
5. The substitution reaction between 2-bromo-2-methylpropane (CH3)3CBr and sodium hydroxide (NaOH) can be described using the following equation: (CH3)3CBr + OH- → (CH3)3COH + Br - It was found that when the concentration of the halogenoalkane was doubled at constant [OH-], the rate of the reaction doubled. When the concentration of NaOH was tripled as constant [(CH3)3CBr], the rate remained constant. |
|||||||||||||
| |||||||||||||
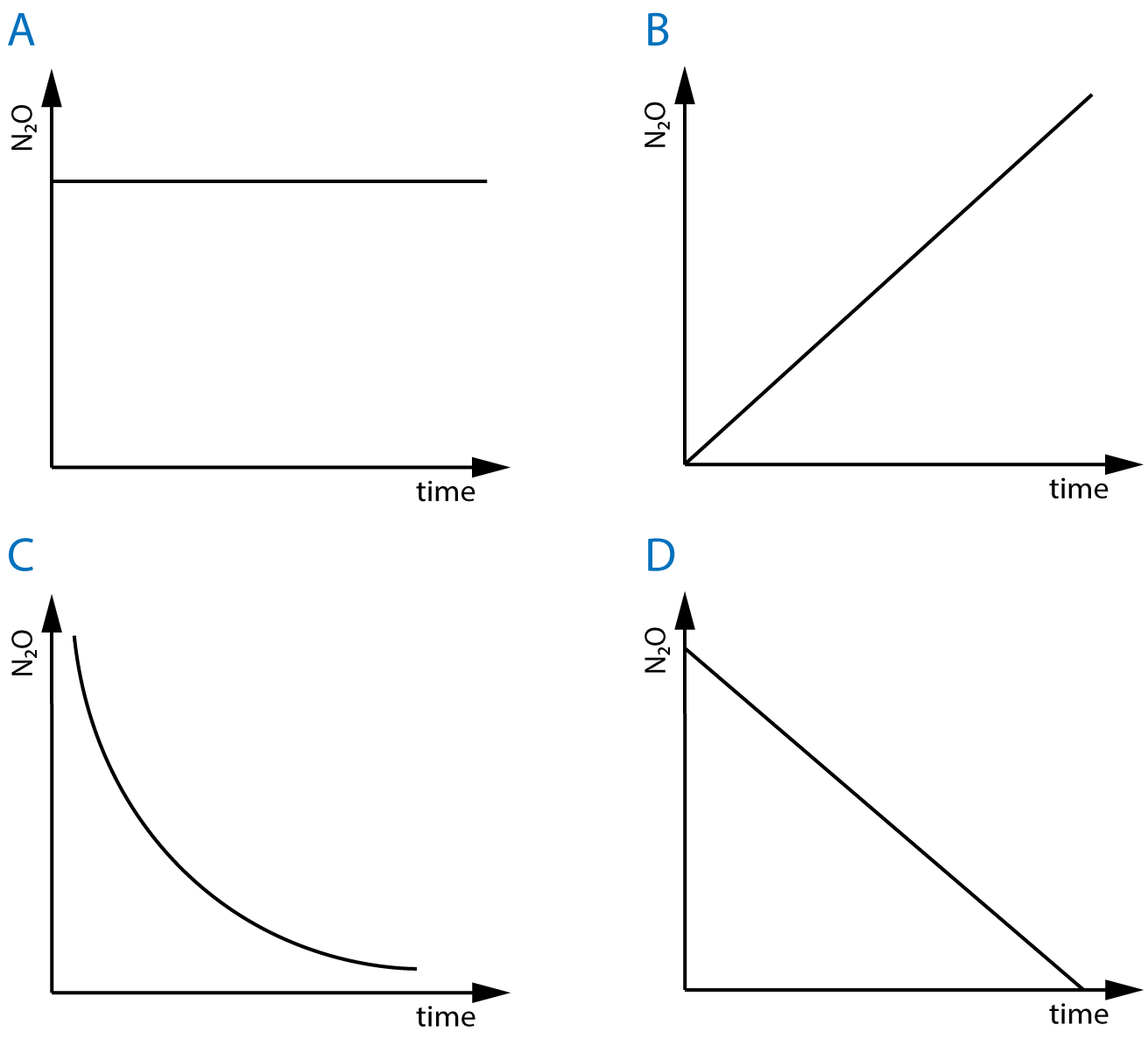
| 6. The decomposition of N2O to N2 and O2 over platinum catalyst is zero order with respect to N2O. Which of the following graphs represent how the concentration of N2O changes over time in this reaction? |
|||||||||||||
 | |||||||||||||
7+8: These questions are about the reaction 2NO + O2 → 2NO2 which has the rate equation Rate = k[O2][NO]2. |
|||||||||||||
7. When the concentration of NO is double and the concentration of O2 is halved, how does the rate of the reaction change?
| |||||||||||||
8. Which of the following is/are possible mechanisms for this reaction?
|
|||||||||||||
| |||||||||||||
| 9. The decomposition of hydrogen peroxide, H2O2, forms H2O and O2:
2H2O2(aq) →2H2O(l) + O2(g) This reaction can be catalysed by potassium iodide, KI, and the rate equation for the catalysed reaction is rate = k[H2O2][I-]. Which of the following statements is correct? |
|||||||||||||
| |||||||||||||
10. Propanone (CH3COCH3) reacts with bromine (Br2) in acidic conditions . Below is an accepted mechanism for this reaction:
Which of the following statements is/are consistent with this mechanism?
|
chemlab Truman University |
||||||||||||
|
|||||||||||||