|
10 minutes maximum An IB Periodic Table is required. |
||||||||||
1. Ethyne reacts with oxygen as shown here: 2C2H2 (g) + 5O2 (g) → 4CO2 (g) + 2H2O(l) Which of the following statements is correct?
| ||||||||||
2. Which one of the following statements is true about endothermic reactions?
|
||||||||||
3. Which of the following equations represent the enthalpy change of formation of propene?
| ||||||||||
4. Which of the following equations represent the enthalpy change of combustion of pentan-1-ol?
| ||||||||||
5. A 27g aluminum block is heated from 18 oC to 68 oC and it absorbs 1215 J of energy. What is the molar specific heat capacity of aluminum in J mol-1 oC-1?
| ||||||||||
6. Which of the following reactions are exothermic?
|
||||||||||
| ||||||||||
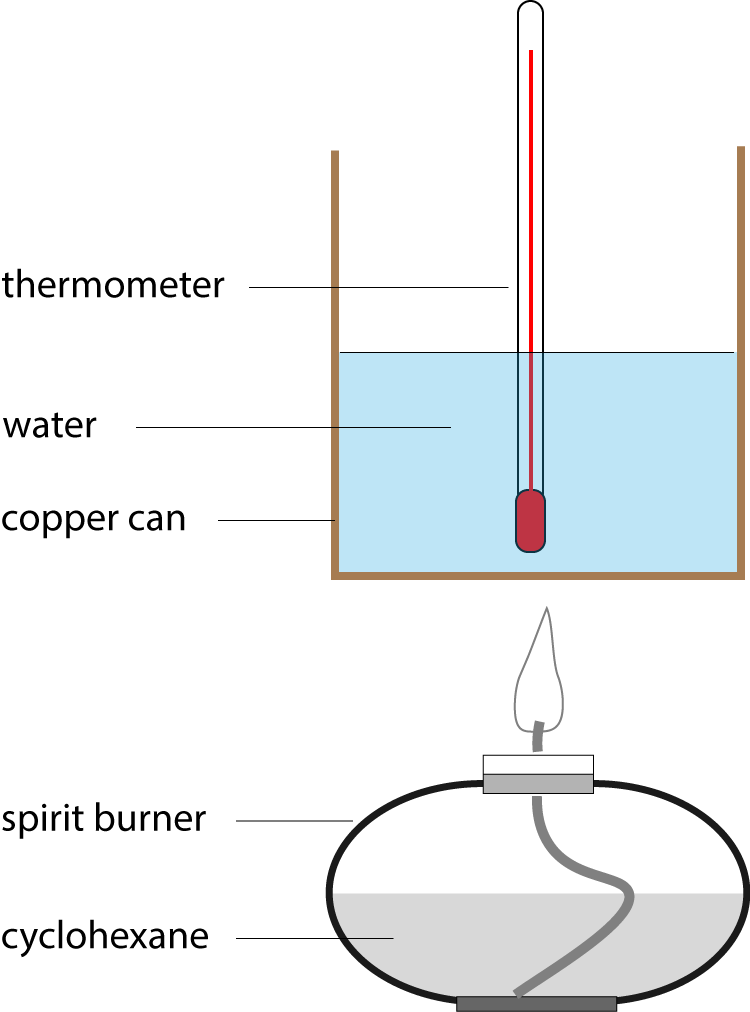
7. An experiment is carried out, as shown in the diagram, to measure the enthalpy change of combustion of cyclohexane. Burning 0.5 g of the fuel increases the temperature of 100g of water by 35 oC. The specific heat capacity of water is 4.18 J g-1 K-1. Which of the following expressions calculates the enthalpy change of combustion of cyclohexane in kJ mol-1? |
 |
|||||||||
| ||||||||||
8. The enthalpy change of solution of ammonium nitrate is given as +25.69 kJ mol-1 in the IB Chemistry data booklet. When 8.0 g of the salt is dissolved in 100 cm3 of water, what is the expected temperature change of the solution in oC? Assume the specific heat capacity of the solution is 4.2 J g-1 oC-1.
| ||||||||||
9. The enthalpy change of the following reaction is -57 kJ mol-1: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) Calculate the amount of heat energy released when 100.0 cm3 of 0.400 mol dm-3 hydrochloric acid reacts with 25.0 cm3 of 0.800 mol dm-3 sodium hydroxide solution.
| ||||||||||
10. 3.00g of zinc powder is measured with a scale and is added into 25cm3 of 1mol dm-3 copper(II) sulphate solution in a glass beaker. The starting temperature and maximum temperature of the reaction are measured using a digital thermometer, in order the calculate the enthalpy change of the reaction. What is the best way to reduce the random error in this experiment?
|
||||||||||