Chemistry µIB:Covalent Structures 1
10 quick questions
|
10 minutes maximum! An IB Periodic Table is required. |
||||||||||||||||
1. Which is the correct Lewis (electron dot) structure for the PCl3 molecule?
| ||||||||||||||||
2. What is the total number of valence electrons in the ethanoate ion, CH3CO2- ?
|
||||||||||||||||
3. Which species is trigonal planar in shape?
| ||||||||||||||||
4. How many electron domains are on the carbon atom in carbon dioxide, CO2?
| ||||||||||||||||
5. What is the shape of the ammonia, NH3, molecule?
| ||||||||||||||||
6. Which row shows the correct electron domain geometry and molecular geometry for the NH2- ion?
| ||||||||||||||||
7. Which statement is NOT true for the H3O+ ion?
| ||||||||||||||||
8. What is the shape of the carbonate ion, CO32-?
| ||||||||||||||||
Q9-10 9. Which is the correct Lewis (electron dot) structure for SF2? |
||||||||||||||||
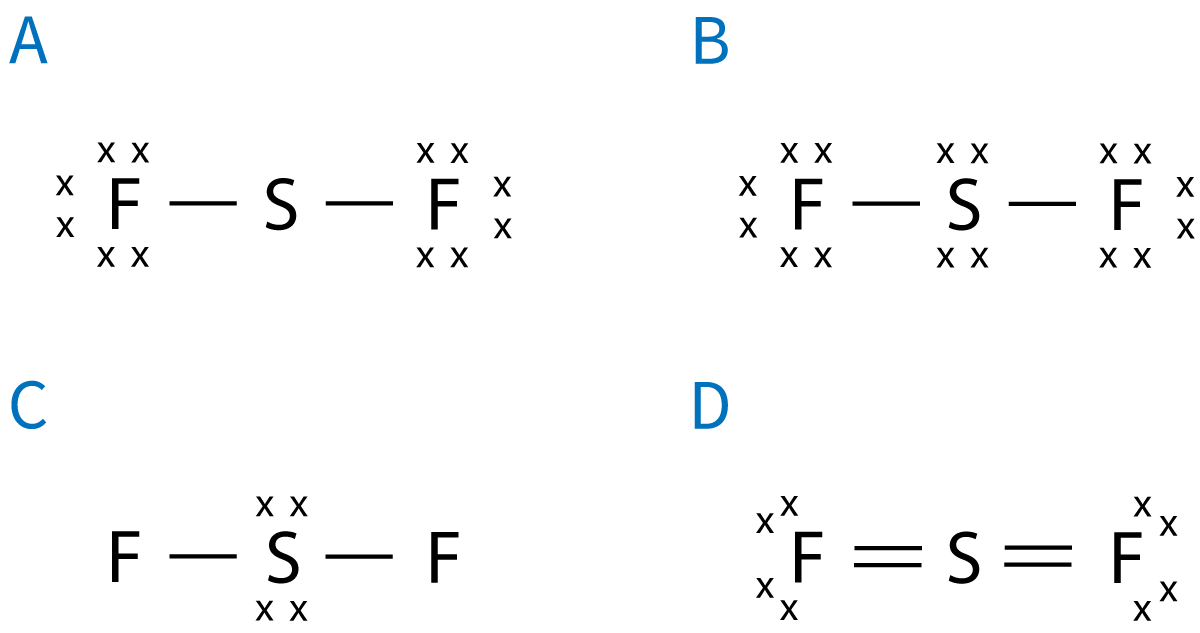 | ||||||||||||||||
10. What is the correct molecular shape for SF2 and will the molecule be polar or non-polar?
|
||||||||||||||||