|
An IB Periodic Table is required |
1. What is the electronic configuration of silicon?
- A. 2,8,6
- B. 2,6,6
- C. 8,6
- D. 2,8,4
| |
2. What are the electronic configurations of the Al3+ and S2- ions?
| |
Al3+ |
S2- |
| A |
2,8,3 |
2,8,6 |
| B |
2,8 |
2,8,8 |
| C |
2,8,6 |
2,8,4 |
| D |
2,8,8 |
2,8 |
|
|
3. Emission spectra are produced when photons are emitted from atoms as ...
- A. excited electrons return to lower energy levels
- B. ground state electrons are excited from lower to higher energy levels
- C. protons and neutrons rearrange themselves in the nucleus
- D. excited electrons move to higher energy levels
| |
4. Which statement is true?
- A. Blue light has a higher energy and lower frequency than red light
- B. Blue light has lower energy and shorter wavelength than red light
- C. Red light has a higher energy and higher frequency than blue light
- D. Red light has a lower energy and longer wavelength then blue light
| |
5. What are the relationships between energy and wavelength, and energy and frequency across the electromagnetic spectrum?
| |
Wavelength |
Frequency |
| A |
Energy ∝ wavelength |
Energy ∝ frequency |
| B |
Energy ∝ wavelength |
Energy ∝ 1/frequency |
| C |
Energy ∝ 1/wavelength |
Energy ∝ frequency |
| D |
Energy ∝ 1/wavelength |
Energy ∝ 1/frequency |
| |
6. Which statement is false?
- A. A continuous spectrum contains all possible frequencies
- B. A line emission spectrum is produced by electrons moving from higher to lower energy levels
- C. Specific frequencies of energy are absorbed in a line emission spectrum
- D. A line spectrum consists of discrete lines of specific wavelengths
| |
Q7+8:
The line emission spectrum of hydrogen in the visible region is shown below:
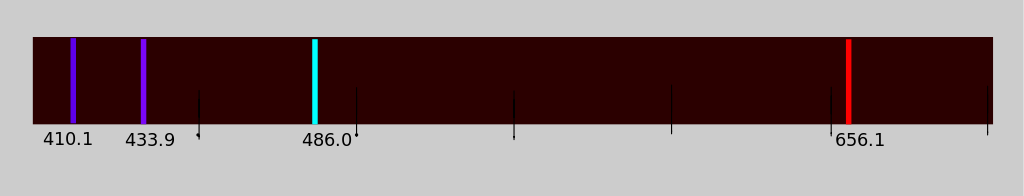
|
Patrick Edward Moran
CC-BY-SA 3.0
|
7. This spectrum provides evidence that ...
- A. Energy levels converge at higher energy
- B. Energy levels are evenly distributed
- C. Energy levels converge at lower energy
- D. Energy levels are randomly arranged
| |
8. Which statement is true for the hydrogen emission spectrum in the visible region?
- A. The lines are produced when electrons move from lower to higher energy levels
- B. The lines are due to electron transitions into the first energy level
- C. The red line represents the greatest energy transition
- D. The lines are due to electron transitions into the second energy level
| |
9. Which electronic transition represents the greatest energy change?
- A. n = 5 to n = 2
- B. n = ∞ to n = 3
- C. n = 3 to n = 2
- D. n = 2 to n = 1
| |
10. What is the correct order of decreasing energy of the following sub-shells?
- A. 4s > 4p > 4d > 4f
- B. 4f > 4d > 4p > 4s
- C. 4d > 4f > 4p > 4s
- D. 4p > 4s > 4f > 4d
|
|
|