11. The following statements relate to the extraction of metals:
| I. Unreactive metals are often found as uncombined elements |
| II. Iron is more difficult to extract than copper |
| III. Sodium is extracted from its ore by heating with carbon |
|
Which of these statements are true?
- A. I and II only
- B. I and III only
- C. II and III only
- D. I, II and III
|
|
Q12-13:
One of the reactions in the extraction of zinc is:
ZnO + C → Zn + CO |
|
12. Carbon is used to extract zinc from zinc oxide because ..
- A. carbon is less reactive than zinc
- B. carbon is more reactive than zinc
- C. carbon is a non-metal
- D. carbon is cheap
|
|
13. Which statement is correct about this reaction?
- A. carbon is reduced to carbon monoxide
- B. carbon monoxide is reduced to carbon
- C. zinc is oxidized to zinc oxide
- D. zinc oxide is reduced to zinc
|
|
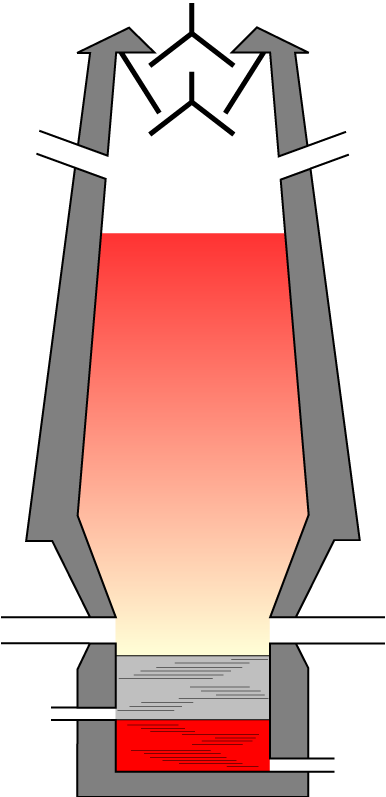
| 14. Iron can be extracted in a blast furnace. The metal oxides are reduced by carbon monoxide, CO.
|
J Navas | CC 3.0
|
The chemical equation for the reduction of iron(III)oxide, Fe2O3 is..
- A. Fe2O3 + CO → Fe2 + CO2
- B. Fe2O3 + 3CO → Fe2 + 3CO2
- C. Fe2O3 + CO → Fe + CO2
- D. Fe2O3 + 3CO → 2Fe + 3CO2
|
|
| 15. Hematite, coke, limestone and hot air are the raw material used in the Blast Furnace.
Which statement is NOT correct about the reactions in the Blast Furnace?
- A. A high temperature is produced when the coke burns in hot air
- B. Limestone decomposes producing carbon monoxide
- C. Iron oxide in the hematite is reduced to iron by carbon monoxide
- D. Impurities in the hematite are converted to slag
|
|
Q16-19:
Aluminium is manufactured by the electrolysis of a molten mixture of aluminium oxide and cryolite using carbon as the positive electrode. |
|
16. Which substances are formed at each electrode?
| |
anode |
cathode |
| A |
aluminium |
oxygen |
| B |
aluminium |
hydrogen |
| C |
oxygen |
aluminium |
| D |
hydrogen |
aluminium |
|
|
17. Why does aluminium need to be extracted from its ore using electrolysis rather than heating with carbon?
- A. Electrolysis is a cheaper process than heating with carbon
- B. Aluminium is more abundant than iron in the Earth’s crust
- C. Aluminium is an expensive metal
- D. Aluminium is more reactive than carbon
|
|
18. Why is a mixture used as the electrolyte rather than pure aluminium oxide?
- A. Cryolite stops the electrodes from corroding away at high temperatures
- B. Aluminium ore is a mixture of aluminium oxide and cryolite
- C. Molten aluminium oxide alone does not conduct electricity
- D. The mixture has a lower melting point than pure aluminium oxide
|
|
19. Why must the positive electrode be continually replaced?
- A. The electrode melts at the temperature used in the process
- B. The aluminium formed reacts with the electrode forming an alloy
- C. The oxygen formed reacts with the electrode forming carbon dioxide
- D. The electrode reacts with the cryolite in the molten mixture
|
|
| 20. Which of the following is a disadvantage of recycling metals? |
 |
- A. less energy is required compared to producing metals from metal ores
- B. fewer quarries and mines are needed
- C. used items require collection, transporting and sorting
- D. supplies of metal ores are preserved
|
|
|
