1. Which of the following statements about conduction of electricity by compounds are true?
| I. All compounds conduct electricity when molten or aqueous |
| II. Covalent compounds do not conduct in any state |
| III. Ionic compounds do not conduct when solid |
|
|
- A. I and II only
- B. I and III only
- C. II and III only
- D. I, II and III
|
|
2.Which of the following statements is true?
- A. Covalent compounds conduct when liquid as molecules are free to move
- B. Ionic compounds conduct in aqueous solution as ions are free to move
- C. Covalent compounds do not conduct when solid as ions are not free to move
- D. Ionic compounds conduct when molten as electrons are free to move
|
|
3. Which is true about the ion attracted to the cathode?
| |
Type of ion |
Charge on ion |
| A |
anion |
positive |
| B |
anion |
negative |
| C |
cation |
positive |
| D |
cation |
negative |
|
|
4. Which of the following substances is an electrolyte?
- A. copper metal
- B. solid sodium chloride
- C. copper(II)chloride solution
- D. molten sugar
|
|
5. Which of these elements could be formed at the anode during the electrolysis of a molten compound?
- A. iodine
- B. copper
- C. hydrogen
- D. sodium
|
|
6. What are the products of electrolysis of molten aluminium oxide ?
| |
Positive electrode |
Negative electrode |
| A |
aluminium |
oxygen |
| B |
aluminium |
hydrogen |
| C |
oxygen |
aluminium |
| D |
hydrogen |
aluminium |
|
|
7. Which statement about the electrolysis of molten lead(II)bromide is correct?
- A. The anode is coated in a brown metal
- B. A brown gas forms at the cathode
- C. Bubbles of colourless gas are seen at the anode
- D. A bead of grey metal forms at the cathode
|
|
| 8. The diagram shows the apparatus for electroplating an iron nail with silver.
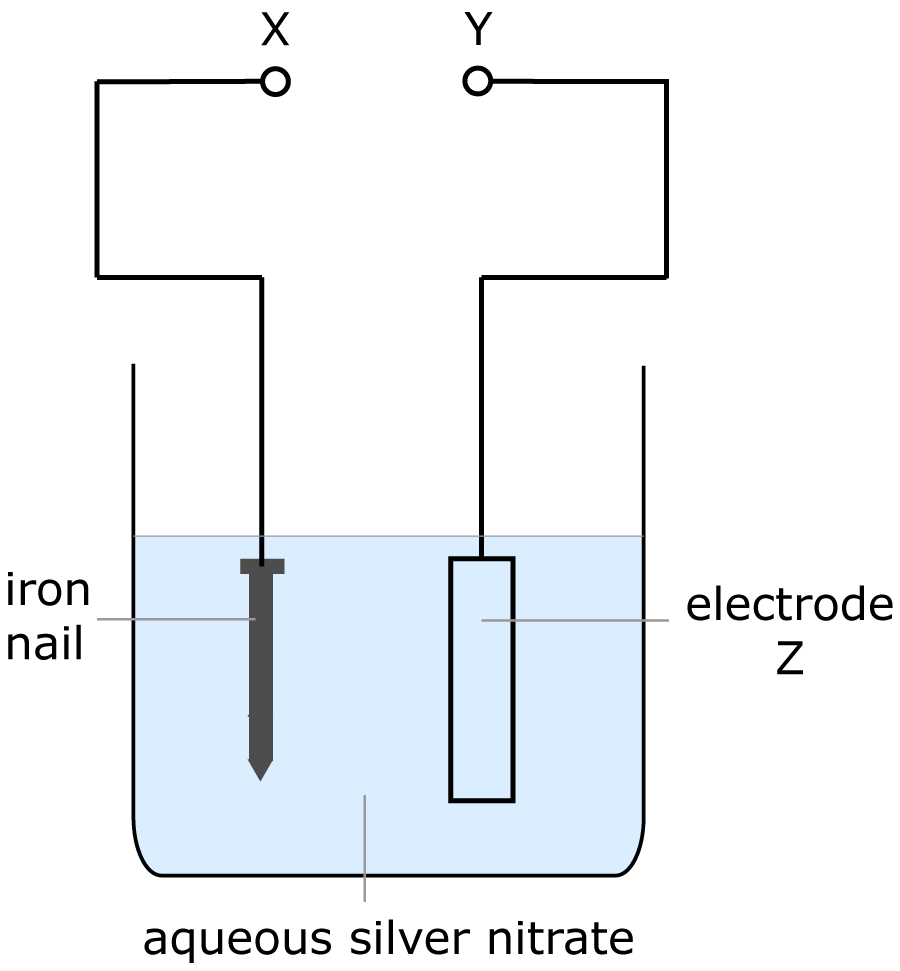
Which row in the table below is correct? |
|
| |
Terminal X is ... |
Electrode Z is made from ... |
| A |
positive |
carbon |
| B |
positive |
silver |
| C |
negative |
carbon |
| D |
negative |
silver |
|
|
9. Molten magnesium chloride was electrolyzed.
Which row gives the correct half-equation for the reaction at the cathode and the type of reaction occurring?
| |
Half-equation |
Type of reaction |
| A |
Mg2+ → Mg + 2e- |
oxidation |
| B |
Mg2+ → Mg + 2e- |
reduction |
| C |
Mg2+ + 2e- → Mg |
oxidation |
| D |
Mg2+ + 2e- → Mg |
reduction |
|
|
|