11. Ethanol can be used as a fuel. The products formed in the complete combustion of ethanol are …
|
|||||||||||||||||
12. Ethanol is oxidized to compound Y when heated with potassium dichromate(VI) in dilute sulfuric acid. The name of compound Y is …
|
|||||||||||||||||
13. Ethanoic acid is a member of the homologous series of carboxylic acids. The structural formula and empirical formula of the fourth member of this series is …
|
|||||||||||||||||
14. Ethanoic acid is regarded as a weak acid because it is …
|
|||||||||||||||||
| 15. Two observation that would be seen when solid sodium carbonate is added to ethanoic acid are … | |||||||||||||||||
|
|||||||||||||||||
| 16. Esters are volatile compounds with distinctive smells and can be used in perfumes. The formula of an ester found in some fruits is shown below:
|
 |
||||||||||||||||
The names of the carboxylic acid and alcohol which reacted together to produce this ester are …
|
|||||||||||||||||
17. Polyesters are condensation polymers produced for use in the garment industry. The structures of two monomers that are used to make polyester are: |
 |
||||||||||||||||
The repeat unit of the polyester produced from these two monomers is: |
|||||||||||||||||
|
|||||||||||||||||
| 18. The molecule shown here is obtained from corn starch and can be used to make a biodegradable polymer, PLA. | 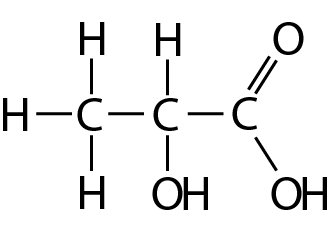 |
||||||||||||||||
What functional groups does this molecule contain?
|
|||||||||||||||||
19. Crude oil can be converted to ethanoic acid as shown in the following flow chart: Crude oil → kerosene → ethene → ethanol → ethanoic acid The four processes used are:
|
|||||||||||||||||
In what order are these processes used?
|
|||||||||||||||||
20. Which of the following is NOT true?
|
|||||||||||||||||