You will need a copy of the periodic table! |
1. Which properties are true for most metals?
- A. high density and high melting point
- B. high density and low melting point
- C. low density and high melting point
- D. low density and low melting point
|
|
2. What type of structure do both diamond and graphite have?
- A. giant ionic
- B. giant covalent
- C. giant metallic
- D. simple molecular
|
|
3. Nitrogen has a low boiling point because there are..
- A. weak covalent bonds between nitrogen atoms
- B. weak covalent bonds between nitrogen molecules
- C. weak forces between nitrogen molecules
- D. weak forces between nitrogen atoms
|
|
4. Rows A to D show the properties of 4 substances.
Which substance is an ionic compound?
| |
Melting point |
Electrical conductivity when molten |
Solubility in water |
| A |
low |
good |
soluble |
| B |
low |
poor |
insoluble |
| C |
high |
good |
soluble |
| D |
high |
poor |
insoluble |
|
|
5. Copper is the metal used in most electrical wiring because it is a very good conductor of electricity.
|

|
Copper is a good conductor of electricity because it ...
- A. contains ions which are free to move
- B. contains atoms which are not free to move
- C. contains delocalized electrons which are free to move
- D. contains delocalized electrons which are not free to move
|
|
Q6-7:
Sodium and fluorine react to form the compound sodium fluoride. |
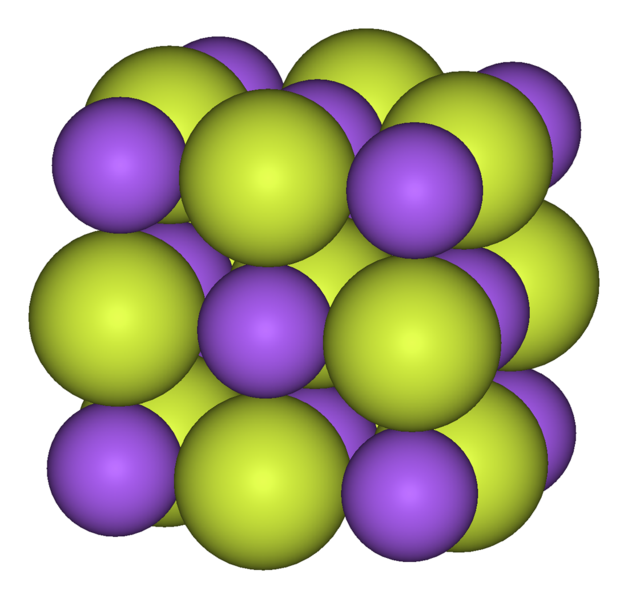
|
| 6. Which statement about the melting point of sodium fluoride is true?
Sodium fluoride has a ……
- A. .. low melting point as the forces between the ions are weak
- B. .. low melting point as the forces between the molecules are weak
- C. .. high melting point as the forces between the ions are strong
- D. .. high melting point as the forces between the atoms are strong
|
|
7. Which statement is true about the conduction of electricity by sodium fluoride?
- A. Sodium fluoride does not conduct in any state
- B. Sodium fluoride conducts when solid or molten
- C. Sodium fluoride conducts when solid or aqueous
- D. Sodium fluoride conducts when molten or aqueous.
|
|
8. Nitrogen and hydrogen gases react to produce ammonia, NH3. Ammonia is a gas at room temperature because ...
- A. it is produced from two gases
- B. there are weak covalent bonds between the nitrogen and hydrogen atoms
- C. it consists of ions with weak attractions between them
- D. it consists of neutral molecules with weak intermolecular forces
|
|
9. Alloys are typically stronger than pure metals because ...
- A. the metallic bonding is stronger in alloys
- B. the layers of metal atoms in the alloy cannot easily slide over each other
- C. alloys are covalently bonded and covalent bonds are strong
- D. the metals atoms are all very regularly arranged in alloys
|
|
|