Triple science only (not for double science students). |
1. Which statement is NOT true about simple cells?
- A. Cells contain chemicals which react to produce electricity
- B. The voltage produced is dependent on the electrolyte used
- C. A battery is two or more cells connected in parallel
- D. The voltage produced is dependent on the type of electrode used
|
|
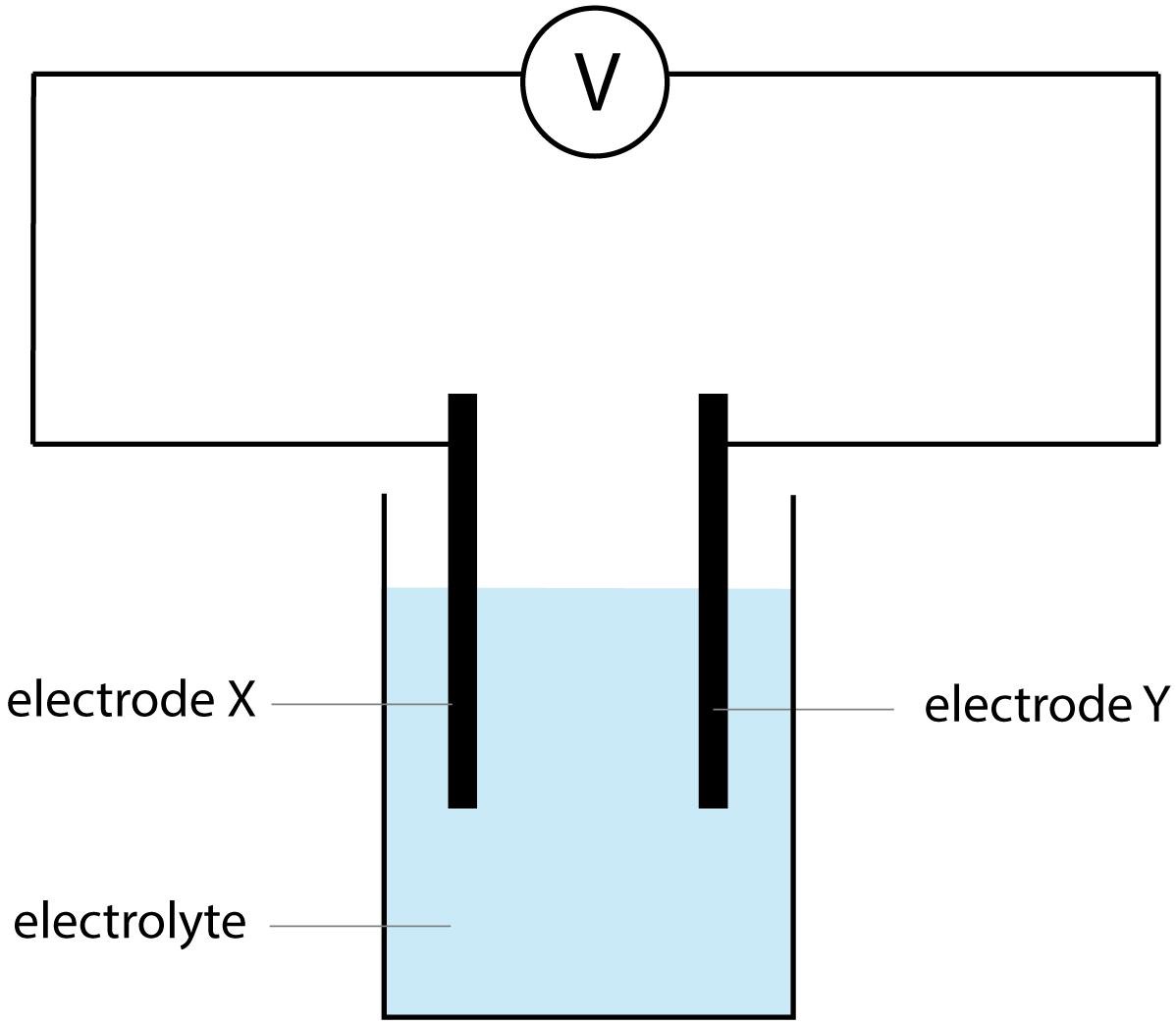
| 2. The diagram shows a simple cell.
Which combination of electrodes and electrolyte would NOT give a reading of zero on the voltmeter? |
 |
| |
Electrode X |
Electrode Y |
Electrolyte |
| A |
Copper |
zinc |
Sodium chloride solution |
| B |
copper |
copper |
Sodium chloride solution |
| C |
Zinc |
copper |
water |
| D |
Zinc |
zinc |
water |
|
|
3. Are alkaline batteries rechargeable or non-rechargeable and why?
| |
Rechargeable? |
Reason |
| A |
Rechargeable |
The chemical reactions are reversible when an external electrical current is supplied |
| B |
Rechargeable |
The chemical reactions are not reversible when an external electrical current is supplied |
| C |
Non-rechargeable |
The chemical reactions are reversible when an external electrical current is supplied |
| D |
Non-rechargeable |
The chemical reactions are not reversible when an external electrical current is supplied |
|
|
4. A non-rechargeable cell stops producing a voltage when ...
- A. the cell has been used for a set amount of time
- B. the cell reaches its ‘use by’ date
- C. one of the reactants runs out
- D. the cell becomes too hot
|
|
5. What is an advantage of a rechargeable cell?
- A. Some of the energy in recharging is wasted as heat
- B. It can only be recharged a finite number of times
- C. It is expensive to manufacture compared to non-rechargeable cells
- D. It reduces the need to mine for more metals and materials
|
|
6. In addition to fuel, what other chemical is need in a fuel cell?
- A. nitrogen
- B. oxygen
- C. petrol
- D. methane
|
|
|
