Chemistry AQA GCSE:Bonding
Page 2
11. A metallic bond is best described as an attraction between ...
|
||||||||||||||||||||||
12. Which of the following is NOT a covalently bonded substance?
|
||||||||||||||||||||||
| 13. Which statement best completes the description of ionic bonding?
Ionic bonding is the strong electrostatic attraction between..
|
||||||||||||||||||||||
14. Sulfur is in group 6 of the Periodic Table. What is the formula of its ion?
|
||||||||||||||||||||||
15. Which dot and cross diagram correctly represents the bonding in an oxygen molecule, O2?
|
||||||||||||||||||||||
16. The diagram shows the outline of part of the Periodic Table: Which two elements could form a covalent compound? |
||||||||||||||||||||||
|
||||||||||||||||||||||
17. The structure of an ionic compound is best described as a lattice structure consisting of a regular arrangement of ..
|
||||||||||||||||||||||
18. Which row is correct about the strength of ionic, covalent and metallic bonds?
|
||||||||||||||||||||||
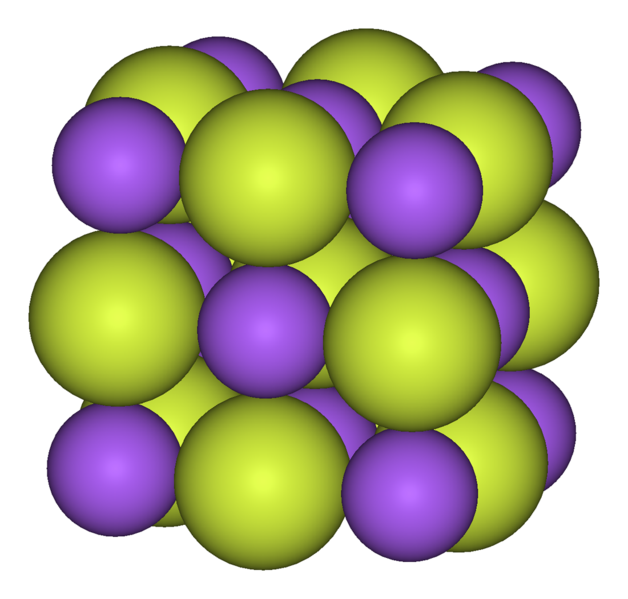
| 19. The picture shows the structure of an ionic compound such as sodium fluoride.
This structure is often described as a ...
|
 |
|||||||||||||||||||||
|
||||||||||||||||||||||
| 20. The diagram shows a ball and stick model of sodium chloride.
What is a limitation of this model?
|
||||||||||||||||||||||
|
||||||||||||||||||||||