Q1-5:
Atoms are made up of three types of particle: proton, neutron and electron. |
| 1. The particle with the smallest mass is the
. |
| 2. The particle with a negative charge is the
. |
3. The particles always present in equal numbers in an atom are …
- A. protons and neutrons
- B. protons and electrons
- C. neutrons and electrons
- D. protons, neutrons and electrons
|
|
4. The particles present in the nucleus of an atom are ….
- A. protons and neutrons
- B. protons and electrons
- C. neutrons and electrons
- D. protons, neutrons and electrons
|
|
5. Isotopes of an element have:
| |
equal numbers of …. |
different numbers of… |
| A |
protons |
neutrons |
| B |
protons |
electrons |
| C |
neutrons |
protons |
| D |
neutrons |
electrons |
|
|
6. The diagram of an atom is shown.
|
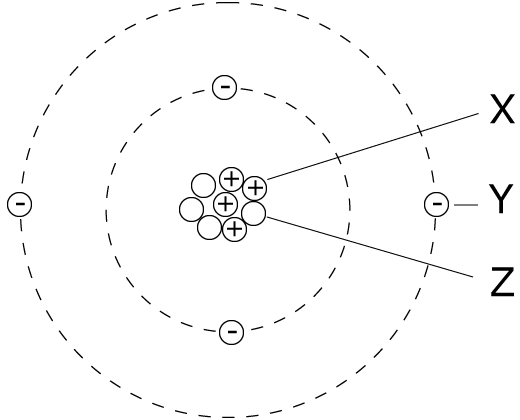
|
Particles X, Y and Z represent:
| |
X |
Y |
Z |
| A |
electron |
proton |
neutron |
| B |
neutron |
electron |
proton |
| C |
proton |
neutron |
electron |
| D |
proton |
electron |
neutron |
|
|
Q7-8:
An atom of magnesium contains 12 protons, 12 electrons and 14 neutrons.
7. What is the electronic configuration of this atom?
- A. 2,8,2
- B. 8,4
- C. 2,8,4
- D. 8,6
|
|
| 8. The mass number of this atom is
. |
| 9. What do the nuclei of |
1 |
H hydrogen atoms contain? |
| 1 |
- A. electrons and neutrons
- B. protons and neutrons
- C. neutrons only
- D. protons only
|
|
Q10-11:
The table shows information on four particles which are atoms or ions.
| |
protons |
neutrons |
Electronic configuration |
| P |
18 |
22 |
2,8,8 |
| Q |
19 |
20 |
2,8,8 |
| R |
19 |
21 |
2,8,8,1 |
| S |
20 |
20 |
2,8,8,2 |
|
|
10. Which particles are ions?
- A. P and Q
- B. R and S
- C. Q only
- D. P, R and S
|
|
11. Which particles belong to the same element?
- A. P and Q
- B. P and S
- C. Q and R
- D. Q and S
|
|
|