NEED HELP? |
  |
|
|||||||||||||||||
How many protons and neutrons are there?
|
|||||||||||||||||
|
2+3: These questions refer to this diagram of an atom of a common element. |
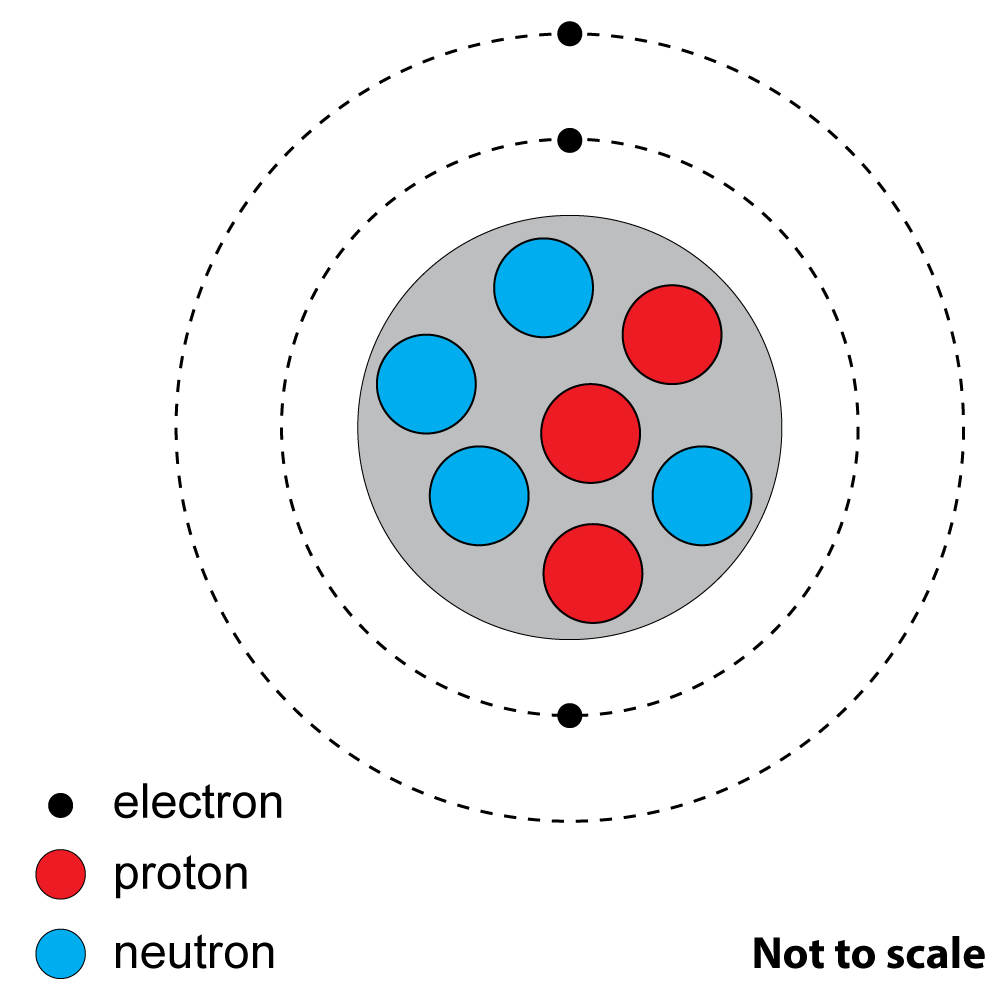 |
||||||||||||||||
2. What is the atomic number? A). 3 |
|||||||||||||||||
| 3. What is the nucleon (mass) number?
A). 3 |
|||||||||||||||||
| 4. Two isotopes of the same element have ...
A). the same number of protons but different numbers of neutrons. |
|||||||||||||||||
| 5. An atom that has been ionised has ...
A). Lost or gained protons |
|||||||||||||||||
| 6. A radioactive isotope is a substance that ...
A). will eventually gain electrons through bonding. |
|||||||||||||||||
7-10:These questions are about types of radiation. Which type(s) of radiation match the following descriptions? |
 |
||||||||||||||||
| 7. The most ionising. | |||||||||||||||||
| 8. A fast moving electron. | |||||||||||||||||
| 9. Can pass through paper. | |||||||||||||||||
| 10.The same as a helium nucleus. | |||||||||||||||||